AMDD –Resolving Device Lag

ADVOCACY
Challenges
- Medical devices and drugs are very different, but were governed by the same regulations and lengthy review times.
- As a result, many medical devices commonly used in other countries remained unavailable in Japan.
Activities
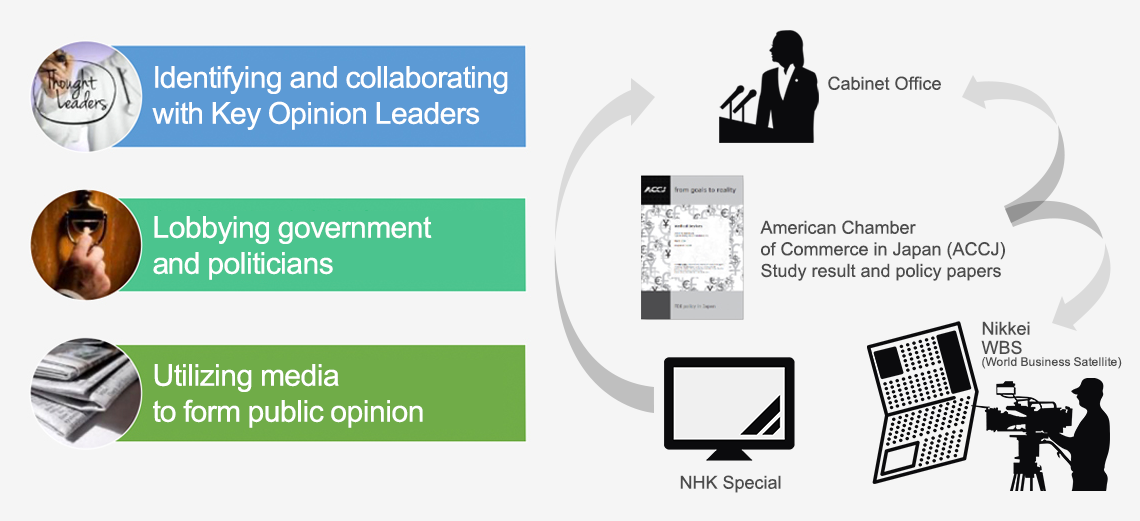
- Sought to build an environment where the world’s latest medical devices may be more easily introduced to Japan for patients to have greater choice when it comes to selecting best treatment.
- Communicated to media the value of medical devices in order to establish a standalone concept of “medical devices” in Japan for the first time.
- Coined the term “device lag” to highlight the issue while communicating public impact.
- Delivered evidence-based awareness-raising activities directly to government and related stakeholders.
Outcomes
Contact us
Back to Case Studies